Radioactivity is a physical phenomenon which occurs when an unstable nucleus of an atom transforms itself through a radioactive decay, reaching a new condition of stability. Radiation can be found everywhere, starting from the first moments of life of our universe. The natural terrestrial radiation is mostly due to three types of decays called α, β and γ, described in the following paragraph.
Radioactive decay is a statistical process: to know when a nucleus will decay into another nucleus is impossible, but is possible to predict the probability of nuclear decay in a unit of time and consequently the mean number of decayed nuclei per second.
Given N0 number of identical nuclei in time t=0, it can be stated that, after a small interval of time Δt, the difference between the number of remaining nuclei and the initial number of nuclei, ΔN is:
where λ is the decay constant [s-1], which is unique for each radionuclide (a radioactive isotope of a chemical element characterized by its atomic number Z, its mass number A, and its nuclear energy state) and expresses the probability that the decay occurs in the unit of time.
This brings to the decay law, that expresses the number of parent nuclei in the system in function of time, shown in the figure below:
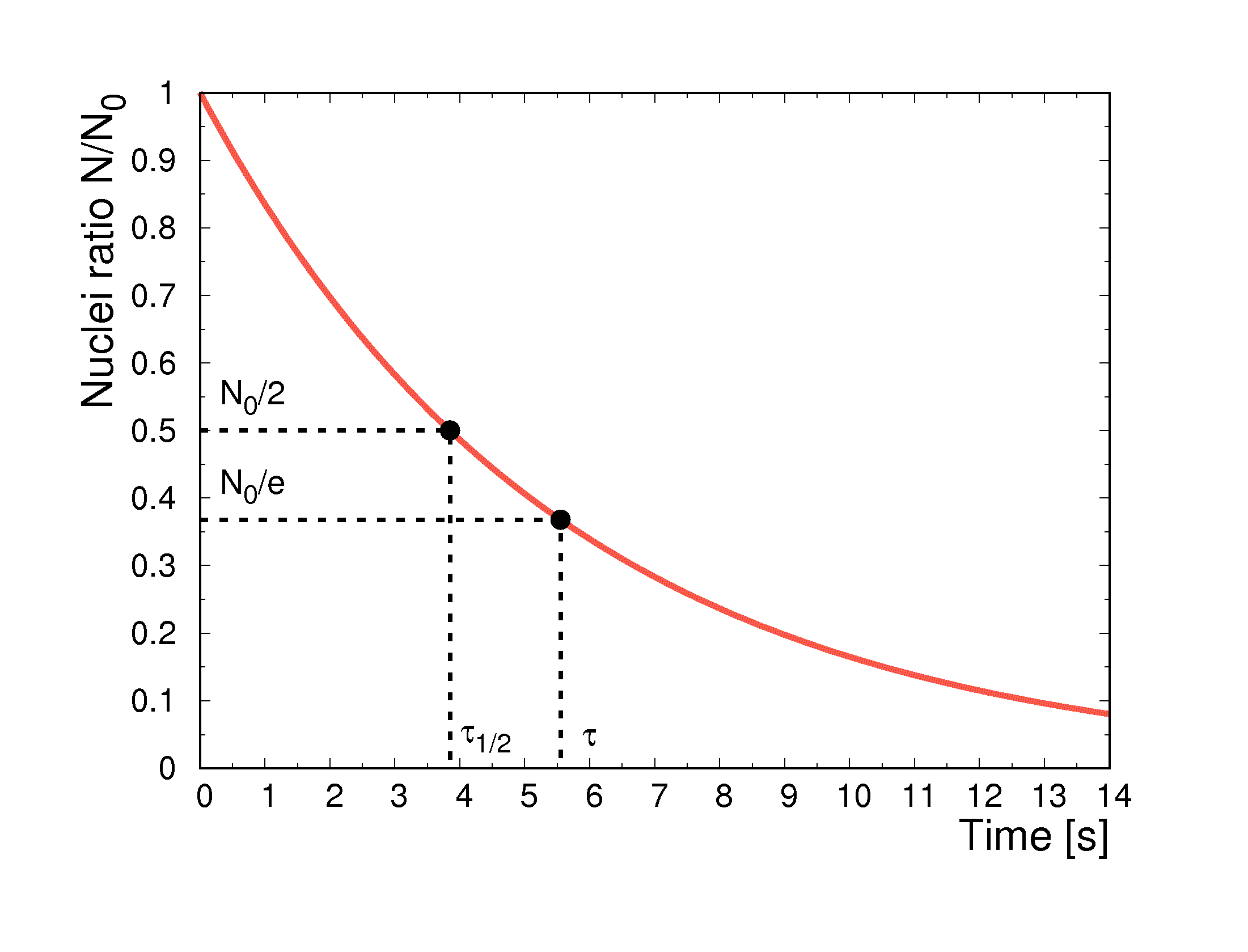
τ, the mean life of a radionuclide, is the required time to reduce the number of nuclei in the system by a factor e and it is the inverse of λ:
Is called half-time t1/2 the time interval in which half of the initial number of nuclei is decayed:
The activity A(t) of a radioactive sample is defined as the number of decays per unit of time:
The activity is measured in becquerels (Bq) defined as one decay per second. We can also define the specific activity: the activity per unit of mass, measured in Bq/kg.
A 4He nucleus is called an α particle and it's composed of two protons and two neutrons. A larger nucleus performs an α decay ejecting an α particle: this is the main type of decay for heavy nuclei (Z > 83). The daughter nucleus then has four unit of mass and two unit of charge less than the parent nucleus:
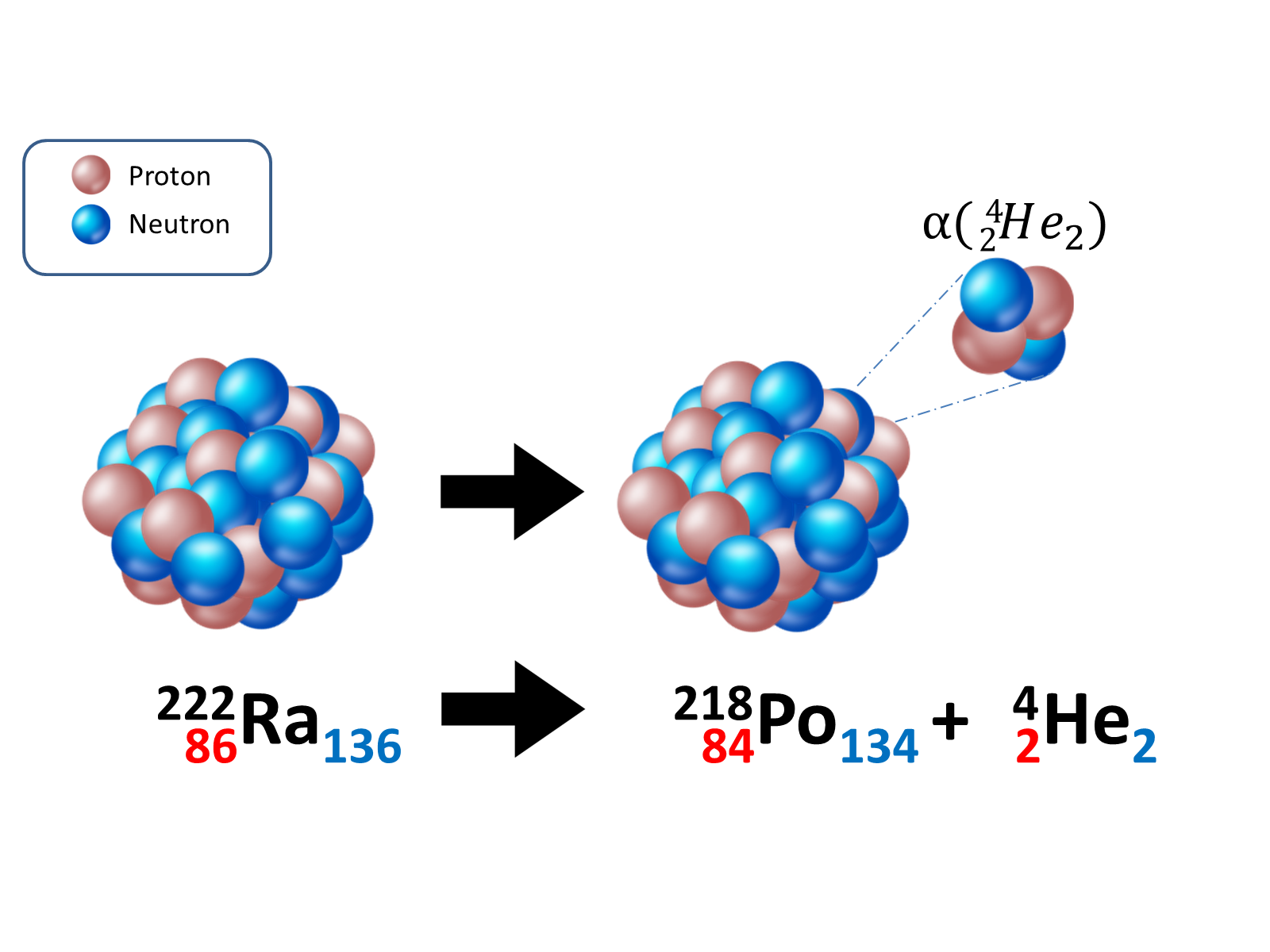
The kinetic energy of the α particle is on the order of MeV, equal to 106 eV, where an eV is 1.602 • 10-19 J.
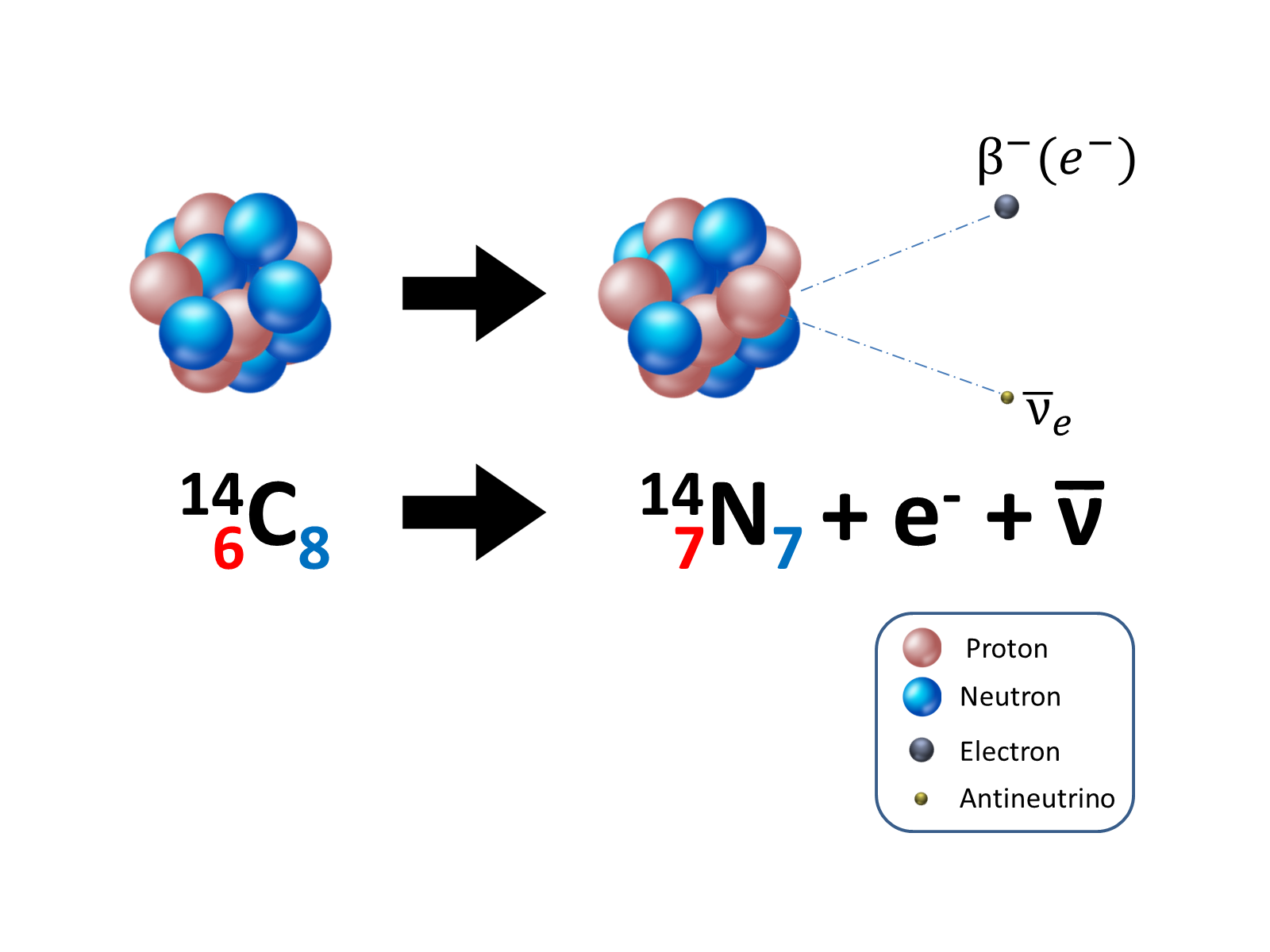
A β decay is the transformation of a neutron inside the nucleus into a proton with the emission of an electron (β- decay) or the transformation of a proton into a neutron with the emission of a positron (β+ decay):
where ve and v̄e are an electronic neutrino and anti-neutrino respectively, according to the laws of conservation of charge and leptonic number.
Nuclei that can decay by β+ can also decay with a process called "electronic capture", described by the following:
While the previous decay modes change the mass number A or the atomic number Z, the γ decay leaves both these numbers unchanged. The nucleus can have many energy levels and α and β decays can leave the daughter nucleus in an excited state. The γ decay occurs when a nucleus in an excited state emits a low (tens of keV) or high energy (1-3 MeV) photon, called a γ, in order to reach a more stable configuration and a lower energy level:
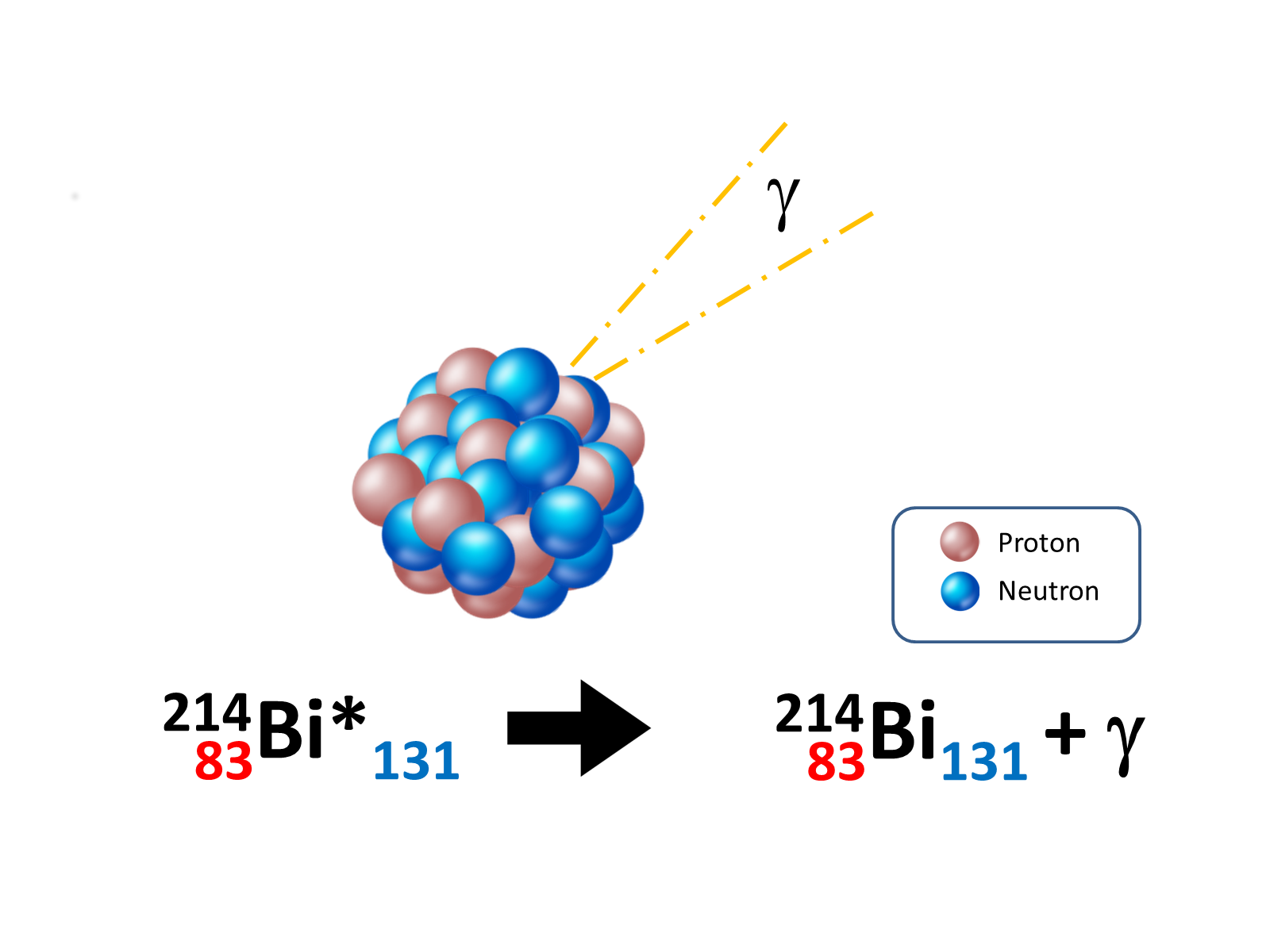
The γ emission following an α or β decay are called αγ and βγ respectively.
Since the energy of a γ must be equal to the difference in energy of the initial and the final nucleus state, the energy spectrum of a γ emitter is discrete, with every line in the spectrum corresponding to a possible shift between two energetic levels of the nucleus.
A radionuclide doesn't always decay directly into a stable nucleus. Radionuclides like 232Th or 238U decay into other radioactive nuclei giving rise to a decay chain in which only the last member is stable.
Each of the decays in a chain has its own decay constant λ. In a closed system, when the atoms of the chain members are not added or subtracted by external agents, provided that the first member of the chain has a longer half-life than its daughters, the activity of every member of the chain is the same:
This condition is called "secular equilibrium" and allows for calculation of the abundance of a parent radionuclide measuring the activity of a daughter.
Main γ-rays emitters in nature
Gamma rays emitted from natural radionuclides, in particular from 238U and 232Th decay chains and from the decay of 40K, are important contributors to the external radiation exposure. The world average radioactivity content in the upper continental crust is 30 Bq/kg, 40 Bq/kg and 720 Bq/kg for 238U, 232Th and 40K, respectively.
Potassium is essential to all living things, including humans, where it's found especially in the muscle tissue. It can be found in most soils, building materials, plants and animals and it is typically used in fertilizers. In nature there are only three isotopes of potassium: 39K (93.3% of weight abundance), 41K (6.7%) and 40K (0.0117%). While, 39K and 41K are stable, 40K is a radioactive isotope with a half-life of 1.28 • 109 years.
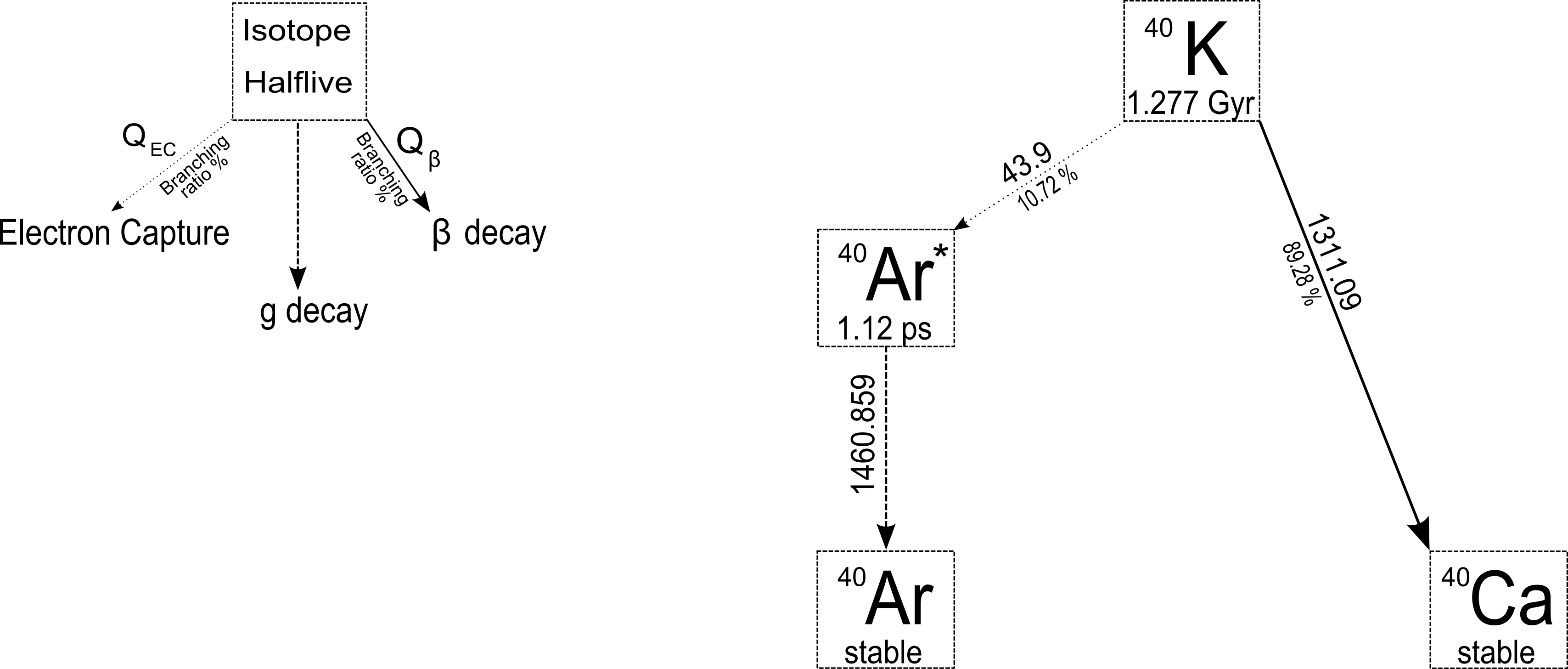
40K decays 89.3% of the time to the ground state of 40Ca by pure β-emission and 10.7% of the time by electron capture to an excited state of 40Ar which then decays γ reaching the stability. The emitted photon has an energy of 1460.86 keV and can be used in order to identify and quantify the activity concentration of 40K in environmental samples.
Naturally occurring thorium is 100% 232Th, an isotope having a half-life of 1.41 • 1010 years, which is greater than the presently accepted age of the solar system. Other isotopes are present in trace amount and are created in long-lived uranium isotopes decay chains or in the 232Th decay chain itself.
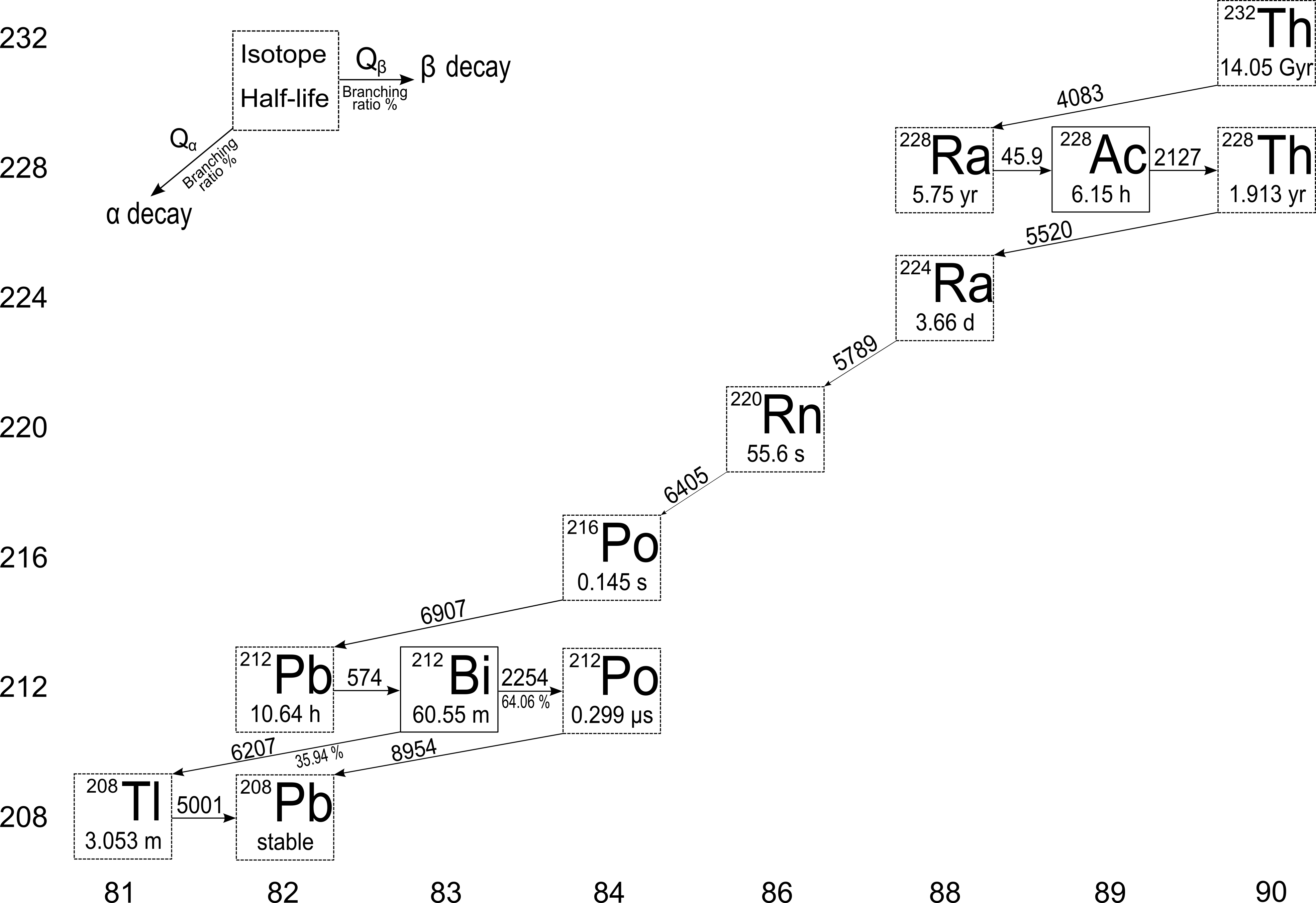
The complex decay chain of 232Th gives rise to several γ decays which complicate the γ spectrum. The most prominent γ emissions arises from the βγ decays of 228Ac (911 keV), 212Pb (239 keV), 212Bi (727 keV) and 208Tl (511 keV, 583 keV, 2614 keV). The relative peak heights may also vary among the environmental samples, depending on the secular equilibrium in the decay chain. If secular equilibrium holds, the activity of each isotope of a decay series is the same. In residues from oil-and gas extraction industry (scale residues) the 232Th and 238U decay chains are generally found in disequilibrium. This happens because radium is chemically a barium-like and calcium-like element and can be found in precipitate that concentrate like those elements.
Like thorium, uranium is found in most building materials and soils on Earth. There are three uranium isotopes that can still be found in the Earth's crust, 238U (99.28% of weight abundance), 235U (0.72%) and 234U (0.0055%). The vast difference in the relative abundances of 238U and 235U is due to the factor 6 of difference in their half-lives, as they are theorized to have been created in nearly equal quantities about 6 • 109 years ago at the time of the supernova explosion that created them. The presence of 234U after this great length of time is due only to its continuous birth in the 238U decay chain.
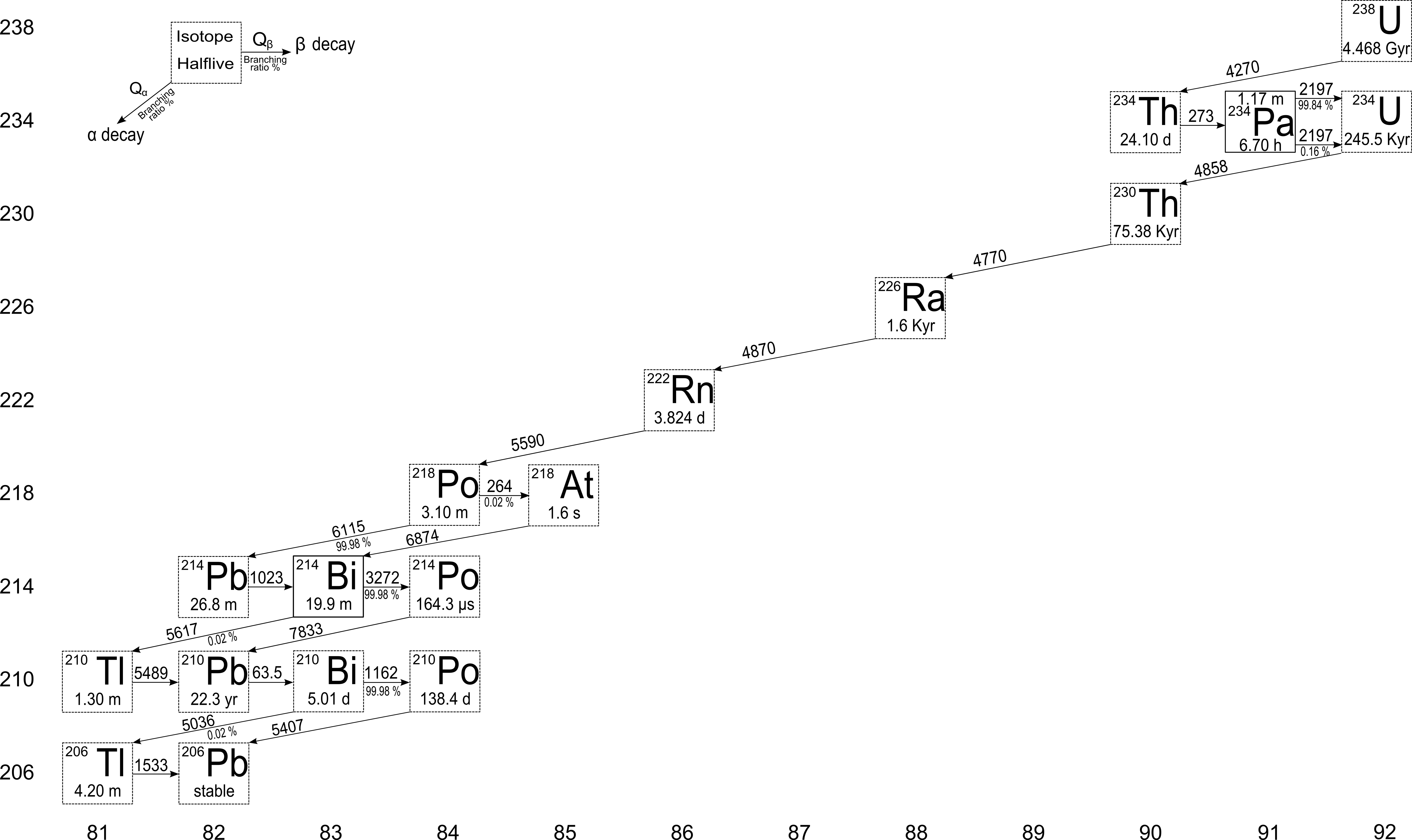
The most prominent gamma emissions in the 238U decays series arise from the βγ decays of 234mPa (1001 keV), 214Pb (295 keV, 352 keV), 214Bi (609 keV, 1120 keV, 1764 keV, 2204 keV, 2447 keV). This radon isotope is the one that worries some health physicists and environmentalists, mostly concerning its concentration in houses, mines, and buildings.






